Covalent molecular compounds exist as liquids or gases at room temperature since they have low melting and boiling points. Ionic bonds are formed between a.
Covalent Compounds Manoa Hawaii Edu Exploringourfluidearth
Molecular compounds of low molecular weight tend to be gases at room temperature.
. Simple molecular substances generally have low melting points and boiling points and are often liquids or gases at room temperature. Melting and boiling are. At room temperature simple molecular substances are gases or liquids or solids with low melting and boiling points.
This explains why many of them are liquids or gases at room temperature. Substances that consist of covalent molecules are usually gases or liquids at room temperature. Many are in the liquid or gas state at room temperature.
This in turns makes it easier to break the bonds that hold each individual atom and more than likely cause them to turn into. Covalent compounds are typically liquids or gases at room temperature although the more complex and the larger the molecule the greater the chance that it could exist as a solid. Which of the following is most likely not a gas at room temperature.
Both of theses molecules have a very small molar mass a very low London dispersion force and thus a very low boiling point. In fact nearly all room-temperature liquids are molecular compounds. For example methane CH4 is a gas at room temperature but octane C8H18 is a.
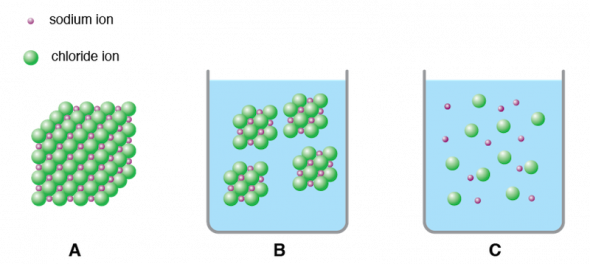
The individual molecules of covalent compounds are more easily separated than the ions in a crystal so most covalent compounds have relatively low boiling points. Even if we assume fully ionic character putting point charges ρ e on molecular centres in the quasi-neutral molecular compound the calculated spontaneous polarization 007 μC. Why do ionic compounds form crystalline solids.
For example molecular compounds that have a very low molar mass like ceCO and ceCH4. To what Celsius temperature must 580 ml of oxygen at 17C be raised to increase its volume to 7000 A. It depends on the temperature.
A single crystalline nanowire of an organic room-temperature-phosphorescent compound E-3. Only two elements are liquid at room temperature and ionic compounds have high melting points. What state of matter is a covalent compound at room temperature.
A 0720 g sample of polyvinylchloride PVC is dissolved in 250 mL of a suitable solvent at 250 C. Water H2O like hydrogen fluoride HF is a polar covalent molecule. What type of covalent bond is H2O.
They are also soft again due. Suppressing molecular motions for enhanced room-temperature phosphorescence of metal. State at room temperature.
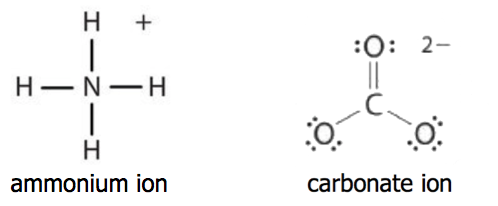
Click to see the answer Q. Covalent bonds consist of pairs of electrons shared by two atoms and bind the atoms in a fixed orientationCovalent Bonds vs Ionic Bonds. It depends on the.
H2 E CH The apparatus pictured below is used to conduct the following experiment. Covalent Bonds Ionic Bonds. At room temperature ionic compounds tend to be solid covalent compounds are more like to be liquids or gases.
After complete evacuation of both chambers valve b is closed and a sample of CO-g is introduced through valve a. The slideshow shows how the weak intermolecular forces between water molecules. At room temperature simple molecular substances are gases or liquids or solids with low melting and boiling points.
At high temperature they meltMolecular covalent compounds. Is a covalent compound at room temperature. Here we report very long quantum coherence times for a transition metal complex of 68 μs at low temperature qubit figure of merit QM3400 and.
Ignoring the effects of pressure the following are true-At room temperature ionic compounds are solids. Most substances with simple molecules have low melting points and boiling points. When the pressure in.
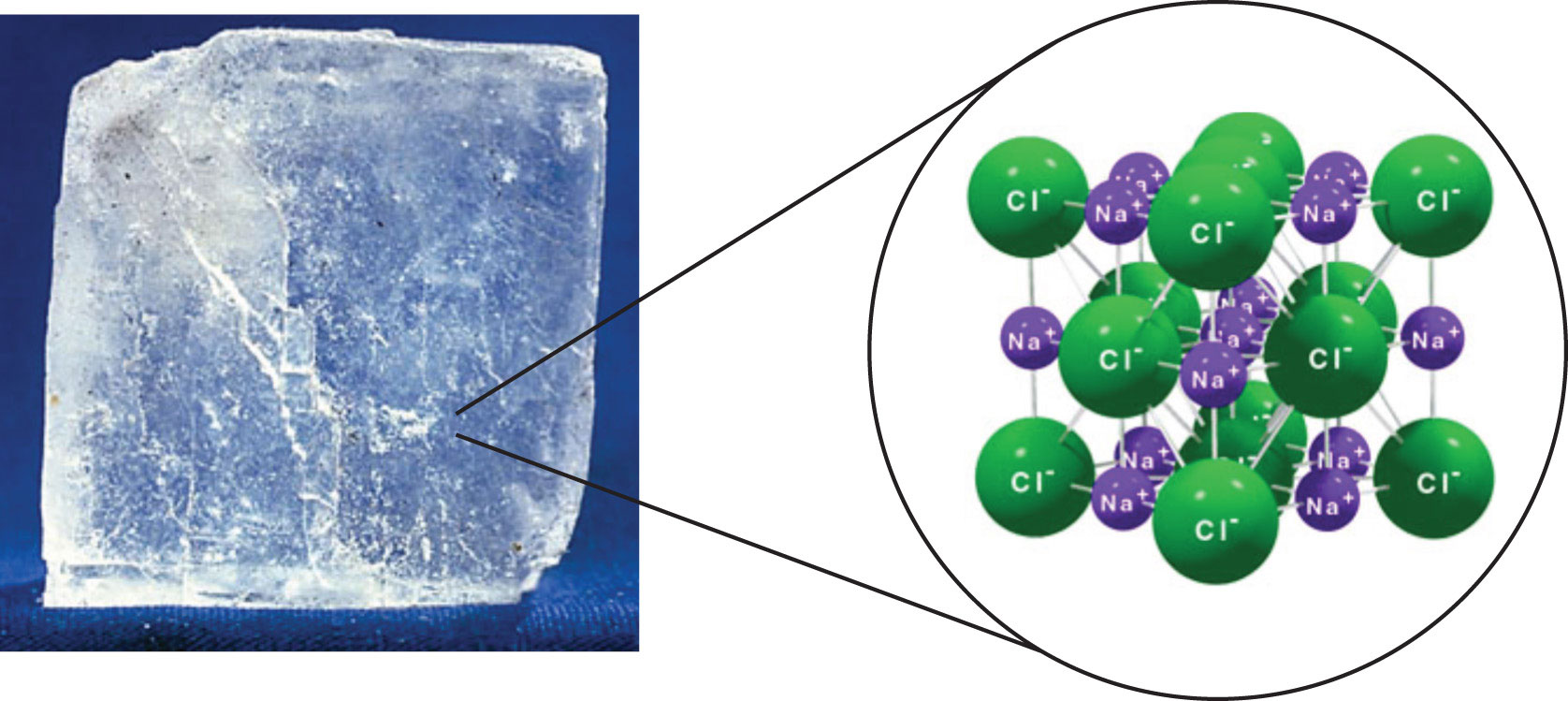
The crystal structure of ionic.
Molecules And Compounds Overview Atomic Structure Article Khan Academy
Molecules And Compounds Overview Atomic Structure Article Khan Academy
4 3 Covalent Compounds Formulas And Names Chemistry Libretexts

Diatomic Elements Molecules Youtube

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts



0 comments
Post a Comment